Identify Each Energy Exchange as Primarily Heat or Work
A book falls to the floor. Identify each energy exchange as primarily heat or work and determine whether the sign of δe is positive or negative for the system.
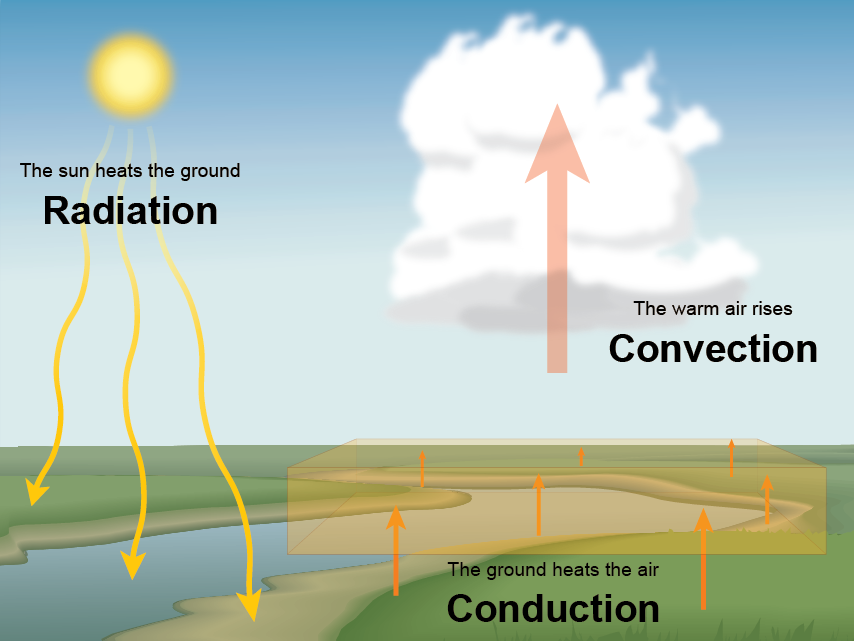
Nws Jetstream The Transfer Of Heat Energy
Conclusively Work done and ΔE are negative.

. Sweat evaporates from skin cooling the skin. Identify each energy exchange as primarily heat or work and determine weather the sign of E is positive or negative for the system. The evaporating sweat is the system b.
The ice cube is the system b A metal cylinder is rolled up a ramp. The first billiard ball defined as the system stops rolling after the collisionb. Identify each energy exchange as primarily heat or work and determine whether the sign of the internal energy Δ𝐸 is positive or negative for the system.
Identify each energy exchange as primarily heat or work and determine weather the sign of ΔE is positive or negative for the system. The first billiard ball defined as the system stops rolling after the collision. The evaporat-ing sweat is the system b.
A book falls to the floor. Identify each energy exchange as primarily heat or work and determine whether the sign of E is positive or negative for the system. The evapo rating sweat is the system b.
At the point when you are holding the object the potential energy will be. Find step-by-step Chemistry solutions and your answer to the following textbook question. The first billiard ball defined as the system stops rolling after the collision.
A 1575-g piece of iron absorbs 108675 joules of heat energy and its temperature changes from 25C to 175C. A gas absorbs 45 kJ of heat and does 29 kJ of work. DE q w where dE is the change in internal energy q is the heat flow and w is the work done.
When the system does work on the surrounding w is -. A work negative b heat positive. A heat engine works through the conversion of energy in the form of heat to chemical work.
Identify each energy exchange as primarily heat or work and determine whether from CHEM 112 at Union County College. A balloon expands against an external pressure. It heat leaves the system q is -.
The metal cylinder is the system. Identify each energy exchange as primarily heat or work and determine whether the sign of ΔE is positive or negative for the sys. If work is done on the system by the surroundings w is.
When heat enters the system q is. The decrease in internal energy takes places for both the system and energy transfer. No work is done by gravity on a bowling ball resting or moving on a bowling alley because the force of gravity on the ball acts perpendicular to the surface.
Sweat evaporates from skin cooling the skin. Answered expert verified. State vs non-state functiona.
Sweat evaporates from skin cooling the skin. The contents of the balloon is the system. Identify each energy exchange as primarily heat or work and determine whether the sign of ΔE is positive or negative for the system.
Identify each energy exchange as primarily heat or work and determine the sign of DE for the system. A An ice cube melts and cools the surrounding beverage. Identify each energy exchange as primarily heat or work and determine whether the sign of Δ E is positive or negative for the system.
1 An ice chest is filled with ice to cool down drinks. Calculate the specific heat capacity of iron. The book is the system.
A balloon expands against an external pressure. Identify each energy exchange as primarily heat or work and determine whether the sign of ΔE is positive or negative for the system. A rolling billiard ball collides with another billiard ball.
A rolling billiard ball collides with another billiard ball. The book is the system. Potential energy and kinetic energy are forms of what kind of energy.
A rolling billiard ball collides with another billiard ball. Identify each energy exchange as primarily heat or work and determine whether the sign of Delta E is positive or negative for the system. The con-tents of the balloon is the system c.
A rolling billiard ball collides with another billiard ball. Note that Δ𝑈 is used as the symbol for internal energy in some sources. A rolling billiard ball collides with another billiard ball.
A book falls to the floor. The first ball system stops rolling after the collision. Identify the outlier in the data set and.
The drinks are the system. Identify each energy exchange as primarily heat or work and determine weather the sign of E is positive or negative for the system. Identify each energy exchange as heat or work and determine whether the sign of heat or work relative to the system is positive or negative.
My answer Work and ΔE is negative for the system. The evaporating sweat is the system b. Determine the temperature change in each.
HELP im stuck on my homework 1. ScienceChemistryQA LibraryIdentify each energy exchange as primarily heat or work and determine whether the sign of ΔE is positive or negative for the systema. Sweat evaporates from skin cooling the skin.

Energy Transformations Scavenger Hunt Energy Transformations Energy Teaching Science

Thermodynamics Part 1 Work Heat Internal Energy And Enthalpy

Endothermic And Exothermic Worksheets And Activities With Answers Exothermic Reaction How To Memorize Things Chemistry Lessons
Comments
Post a Comment